One hundred years ago, people were just coming to grips with a strange idea: Everything is made of atoms.
The notion had been around for a very long time. But all of a sudden a truckload of evidence emerged that it was really true. Even though common sense would seem to shout otherwise, reality is composed of exceedingly tiny particles.
The world appears to be composed of large, solid things: begonias, mockingbirds, banjoes, mountain ranges, and Nebraska.
But everything we know and experience, including the air we breathe and the stars we see in the night sky, is actually atomic. Each atom has a nucleus containing a certain number of protons and neutrons that is orbited by the same number of electrons.
The atomic basis of reality is now an unquestioned principle of physics. But that doesn’t eliminate its sheer strangeness.
Atoms, for instance, are astonishingly small. As Bill Bryson explains in A Short History of Nearly Everything: “Half a million of them lined up shoulder to shoulder could hide behind a human hair.”
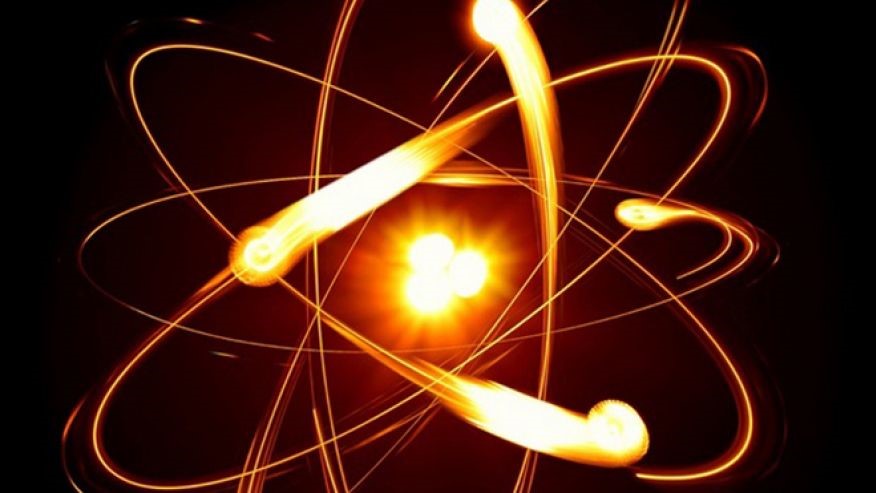
They are notoriously hard to visualize. Early in the 20th century a picture was proposed that has become lodged in our collective imagination: An atom looks like a miniature solar system, with electrons zinging around the nucleus like tiny planets. Note, for instance, the illustration above.
Physicists now know that such a picture is misleading. It’s more accurate to visualize the electrons as a cloud or a blur, like the blades of an incredibly high-speed fan. The German physicist Werner Heisenberg, when asked how we should picture a typical atom, replied, “Don’t try!”
Atoms are mostly empty space. William H. Cropper suggests that if an atom were expanded to the size of a cathedral, the nucleus would be about the size of a house fly – but a fly that weighs hundreds of thousands times more than the cathedral.
Common sense tells us that when two objects come together, they strike each other. Think of two NFL players colliding at the goal line, or your two hands clapping enthusiastically up in Section 38.
Scientists, however, point out that “solid” objects never actually touch each other at all. The negatively charged electron fields of adjoining atoms push back on each other. Thus when you go driving later today, your body will actually be levitating about a hundred millionth of a centimeter above the driver’s seat, and your car’s tires will be oh-so-close but not actually in contact with anything solid on the street. “Contact” is an illusion that we choose to believe.
Perhaps strangest of all, atoms last a long time. Even though we never feel it, the atoms that make up our bodies are constantly being replaced.
It’s estimated that the 1027 atoms in an average human being (that would be 10 followed by 27 zeroes – a very big number) are swapped out every seven years or so. Thus if you’ve ever thought that you’re not quite the same person that you were in 2015, it’s because you quite literally aren’t.
The atoms that used to hang out in your body may go away, but they don’t disappear. They’re just hanging out somewhere else. The hydrogen and oxygen atoms that make up the water you will drink today, for instance, were no doubt once drunk by a Stegosaurus. Let’s not even think about dinosaur pee.
Physicists state confidently that each of us is currently “home” to a number of atoms that have been part of virtually any historical figure you can name. About a billion of your atoms were at one time or another a part of St. Francis of Assisi, Mary Queen of Scots, Genghis Khan, Cleopatra, George Washington Carver, Babe Ruth, and Eleanor Roosevelt. Elvis may have left the building, but he left his atoms behind. It takes a little longer for the atomic recycling of the recently deceased, but it’s likely that each of us right now owns a little bit of what used to be the King.
Which brings us to a much more interesting and redemptive observation. At least one billion of your atoms were once part of the King of the Universe, a Jewish carpenter named Jesus of Nazareth.
Here’s where the New Testament exceeds the strangeness of atomic physics. “So the Word of God [Jesus] became a human being and lived among us” (John 1:14). Did God actually become the same material that makes up the rest of the cosmos – including us?
The cornerstone of Jesus’ teaching is that he is both human and divine – beyond this world, yet also of this world. In a way we cannot comprehend, God the Son became part of God’s own creation.
Common sense tells us that God just doesn’t do things like that.
The Gospel of Matthew tells us, on the other hand, that Jesus is called Immanuel. That means “God With Us.”
Which means that our Savior is pleased, even at this very moment, to share the very same stuff of which we are made.
